ENGOT-0V50/INNOVATE-3 – Ovarian Cancer Clinical Trial
overview
The ENGOT-ov50/INNOVATE-3 study is a pivotal (like a Phase 3), randomized, open label study. This clinical study is evaluating the safety and efficacy of Tumor Treating Fields (TTFields), delivered by the NovoTTF-200(O) device concomitant with a standard chemotherapy agent paclitaxel, for patients recently diagnosed with recurrent ovarian cancer.
This study has completed its enrollment. Please go to find a trial to search other options in your area, or contact us.
This study will randomize patients 1:1 into one of two treatment groups: TTFields treatment together with paclitaxel, or treatment with paclitaxel alone.[The ENGOT-ov50/INNOVATE-3 study].
Novocure GmbH, the study sponsor, maintains this website to help patients get basic information about the study.
about TTFields
Tumor Treating Fields (TTFields) are electric fields that disrupt cancer cell division [Science of TTFields]. TTFields may interfere with the electrically charged cellular components of cancer cells, disrupting their normal function and may ultimately lead to cell death. As a result, the cancer cell division may be slowed or even stopped, inhibiting tumor growth.
TTFields are delivered to the body region where the tumor is located using an investigational medical device called NovoTTF-200(O). NovoTTF-200(O) is a portable, lightweight, battery-operated device designed to deliver TTFields to recurrent ovarian cancer.
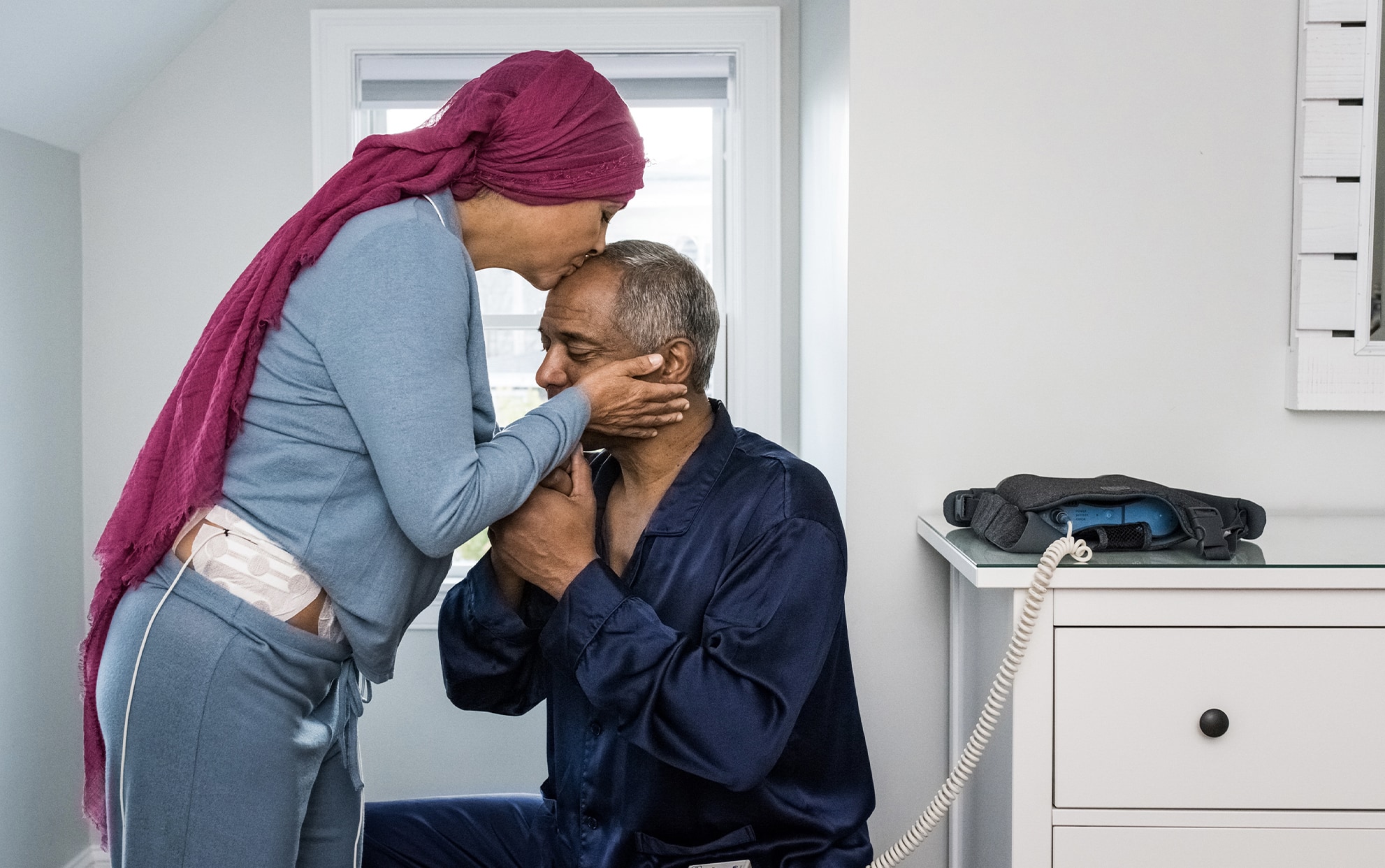
Patients receiving TTFields need to wear four adhesive patches called ILE Transducer Arrays (arrays) on their abdomen and pelvis, which deliver the TTFields non-invasively to the cancer site. The device is intended for continuous home use by patients [Living with TTFields].
NovoTTF-200(O) for the treatment of recurrent ovarian cancer is investigational; it has not been approved by the U.S. Food and Drug Administration (FDA) or other regulatory authorities. The safety and effectiveness of TTFields in this condition is still unknown, and will be evaluated in this clinical study [The ENGOT-OV50/INNOVATE-3 Study].
TTFields therapy has been approved for adult patients with recurrent (as a monotherapy) and newly diagnosed glioblastoma multiforme (GBM) (in combination with a chemotherapy called temozolomide), in the United States under the Premarket Approval (PMA) pathway, has obtained a CE mark in Europe, and is approved in Japan for the same indication. The use of TTFields concurrent with chemotherapy has also been CE marked in Europe and approved for adult patients with malignant pleural mesothelioma (MPM) under FDA’s Humanitarian Device Exemption (HDE) pathway.
In May 2020, the China National Medical Products Administration (NMPA) approved Optune® for the treatment of newly diagnosed and recurrent glioblastoma. In August 2020, Optune Lua™ launched for the treatment of malignant pleural mesothelioma (MPM) in Hong Kong.
the ENGOT-OV50/INNOVATE-3 Trial
The ENGOT-ov50/INNOVATE-3 study is pivotal, randomized, open-label study of Tumor Treating Fields (TTFields, 200kHz), generated by the medical device NovoTTF-200(O) [About TTFields]. Study patients use the device concomitant with weekly paclitaxel for treatment of recurrent ovarian cancer. The study is expected to enroll 540 patients in centers around the world [ENGOT-OV50/INNOVATE-3 Centers].
The ENGOT-OV50/INNOVATE-3 Study Design
The ENGOT-ov50/Innovate-3 study is for patients recently diagnosed with recurrent ovarian cancer. Final eligibility can only be determined by the clinical study doctor at one of the clinical study centers. All patients enrolled in the study will receive a standard of care treatment for their ovarian cancer, paclitaxel, similar to patients who are not participating in the study. All patients will be randomly assigned to one of two groups:
- The TTFields Group: Patients will receive standard chemotherapy (paclitaxel) in combination with TTFields.
- The Standard of Care Group (control): Patients will receive standard chemotherapy (paclitaxel) alone.
Each patient will have a 50% chance of entering each of the two groups as determined by a “randomization process” (similar to a coin flip). Eligible patients will be randomized within 28 days of signing an Informed Consent Form for the clinical study.
Regardless of which treatment arm patients are assigned to, they will receive weekly paclitaxel as standard chemotherapy. After the first 8 weeks, paclitaxel will be administered weekly for 3 weeks out of every 4 week (28 day) cycle.
Using the Device
For those patients randomly assigned to the TTFields group, to receive TTFields treatment the patient wears four arrays on their abdomen and pelvis which deliver the TTFields non-invasively to the tumor location. Array placement may require shaving of the contact site before starting to use the device, and 2-3 times a week for as long as its use continues. The arrays are replaced and re-applied on a regular basis for as long as the patient is receiving the treatment.
The use of NovoTTF-200(O) will include training on the operation of the equipment by a qualified Device Support Specialist (DSS). Patients will continue to use the device at home [Living with TTFields].
Once trained, patients using NovoTTF-200(O) can setup and use the device themselves (with the help of a family member or a caregiver, if needed), or may receive support from their DSS.
The device is designed to be user-friendly and all technical aspects related to using it are maintained by the DSS. The DSS can also help patients in finding technical solutions to allow each individual to receive TTFields while maintaining their normal daily routines.
Eligibility
For the complete list of eligibility criteria please go to clinicaltrials.gov or consult with an ENGOT-ov50/INNOVATE-3 study physician:
Key Inclusion Criteria (summary, not complete list):
- 18 years of age and older
- Histologically confirmed diagnosis of ovarian carcinoma that has not responded to therapy containing platinum within 6 months of last treatment with platinum
- Life expectancy of ≥ 12 weeks
- Amenable to receive weekly paclitaxel and able to operate the NovoTTF-200(O) device
- Signed informed consent form for the study protocol
Key Exclusion Criteria (summary, not complete list):
- History of disease progression on a weekly paclitaxel regimen
- Spread of the cancer to the brain
- Albumin level <25 gram/liter
- Grade 3 or higher peripheral neuropathy
- Implantable electrical medical devices
- Known allergies to medical adhesives or hydrogel
- Known reaction to paclitaxel or drugs similar or related to paclitaxel
- Prior cancers treated for recurrence within 2 years, except for completely resected non-melanomatous skin carcinoma, or successfully treated in situ carcinoma of the skin, breast or cervix of the uterus
- Serious co-morbidities
- Concurrent anti-tumor therapy beyond weekly paclitaxel, excluding hormonal therapy for breast cancer
- Concurrent active treatment in another clinical study. However prior participation in clinical study is allowed as well as participation during survival follow-up
- Pregnancy or breast-feeding
- Admitted to an institution by administrative or court order
Enrollment
This study has completed its enrollment. Please go to find a trial to search other options in your area, or contact us.
Study Costs
There will be no cost to participate in this study. The study sponsor (Novocure) will pay for reasonable treatment-related supplies, including the NovoTTF-200(O) device and accessories. Patients and their insurance will be responsible for medical treatments received in the normal course of patient care*. The study sponsor may also reimburse patients for reasonable travel costs directly related to the study’s procedures. Such reimbursements are done according to the policy of the study center at which the patient is being treated. Prior to enrolling in the study, please consult with the clinical study team at the center about the local reimbursement policy and your rights.
*Some regional differences may apply. Please contact the study center closest to you for more information.
ENGOT-ov50/INNOVATE-3 centers
This study has completed its enrollment.
Please go to find a trial to search other options in your area, or contact us.
living with TTFields
Patients participating in the ENGOT-OV50/INNOVATE-3 study use the device continuously for an average of at least 18 hours a day, but may take breaks during daily use for some activities. The treating physician may modify the treatment schedule and use of the device as needed.
The DSS will provide support to patients and caregivers.
Please note: While the DSS provides comprehensive technical support, direct all medical questions to your treating physician.
Using NovoTTF-200(O)
NovoTTF-200(O) is intended for continuous use for at least 18 hours a day. The device should not interfere with household or personal electrical devices. There is no exposure of TTFields to non-users, such as co-workers and family members.
What does it look like?
NovoTTF-200(O) is a wearable, portable device. Use of the device requires shaving of the contact site and the application of adhesive patches called arrays to the abdomen and pelvis. Naturally, some patients may be concerned about their appearance or their lifestyle while using the device. The arrays and the wires connecting to the device can be concealed underneath the patient’s clothes. By doing so, the bag carrying the device may be the only element of NovoTTF-200(O) visible to others. The medical team will provide personalized assistance specific to each patient for wearing the device comfortably.
In addition to regularly shaving the contact area, patients will regularly change their arrays to ensure continuous treatment. The medical team will assist in creating a plan and instructing patients on array placement.
Portability
The device gives patients the option to maintain their lifestyle without interference to their normal daily routine. Consult the treating physician ahead of time regarding any activity in question. In addition, the DSS can provide information to address lifestyle concerns.
The device bag can be worn as a shoulder bag, a backpack, a messenger bag, or hand-held. The total weight of the device including a battery is around 1.3 kilograms, or 2.7 pounds. Novocure can provide as many batteries as needed.
In order to ensure continuous treatment, patients may plug NovoTTF-200(O) into a wall outlet when staying in a location for more than a few hours.
science of TTFields
Cancer cells divide and multiply rapidly within the ovarian cancer tumor. These cancer cells carry different types of electrically charged elements that play a role during the cell division process. Other healthy cells in the treatment area multiply at a much slower rate, if at all.
TTFields Mechanism of Action
NovoTTF-200(O) delivers low intensity, wave-like electric fields to the location of the tumor. These fields are known as Tumor Treating Fields (TTFields).
Pre-clinical studies showed that due to the shape and size of cancer cells when they are multiplying, TTFields cause electrically-charged cellular components of these cells to change their location within the dividing cell, disrupting their normal function and may ultimately lead to cell death.
In addition, cancer cells also contain miniature building blocks that act as tiny motors in moving essential parts of the cells from place-to-place. TTFields interfere with the normal orientation of these tiny motors since they are also electrically charged. As a result, the cancer cell division is slowed, or even stopped, inhibiting tumor growth.
A summary of TTFields’ mechanism of action is illustrated in the following animation:
TTFIELDS ARE NOT APPROVED FOR THE TREATMENT OF RECURRENT OVARIAN CANCER. THE SAFETY AND EFFICACY OF TTFIELDS FOR THIS INDICATION HAS NOT BEEN ESTABLISHED.
Past Clinical Experience
To date, the sponsor has investigated a number of similar devices developed to deliver TTFields to different tumors. The main difference between the previously tested devices and NovoTTF-200(O) is the frequency of TTFields provided by each device. The frequency is adjusted by the manufacturer of the device in order to optimize the treatment for each treated cancer, based on data from laboratory experiments.
NovoTTF-100L(O), a previous model of the NovoTTF-200(O) device, has been evaluated in the INNOVATE pilot study of 31 patients platinum-resistant ovarian cancer (PROC) patients who received TTFields in combination with paclitaxel to test the safety of TTFields in this indication.
A similar device, the NovoTTF-200A (also called Optune®), delivers TTFields to the brain and is approved by the FDA for the treatment of adults with recurrent and newly diagnosed glioblastoma, a type of aggressive primary brain tumor. Optune has a CE (European conformity) Mark in Europe for the treatment of glioblastoma, and is also now approved in Japan. For the treatment of malignant pleural mesothelioma (MPM), Optune Lua™ has a CE Mark in Europe and was FDA approved under the Humanitarian Device Exemption (HDE) pathway for this use.
Side Effects
Based on the science behind TTFields and the clinical results to date, NovoTTF-200(O) is not expected to have systemic side effects in patients with recurrent ovarian cancer. In previous studies performed with Optune, the device tested in glioblastoma, a high percentage of patients have experienced local skin irritation beneath the transducer arrays, which was mild to moderate in severity in the vast majority of cases.
In the aforementioned pilot study for PROC, there were no serious systemic side effects attributed by the study doctors to TTFields. The most reported side effect to the device was mild to moderate skin irritation under the arrays placed on the skin.
The information provided in this website is partial, and you should consult with your treating physician concerning the complete safety profile of TTFields.
FAQ
What is the objective of this study?
This pivotal study is designed to test the safety and efficacy of TTFields treatment together with standard chemotherapy agent paclitaxel, for the treatment of recurrent ovarian cancer.
How can I participate in the ENGOT-ov50/INNOVATE-3 study?
This study has completed its enrollment.
Please go to find a trial to search other options in your area, or contact us.
What are the expected side effects of TTFields?
The use of NovoTTF-200(O) is not expected to have systemic side effects, based on our clinical data to date. Some patients have experienced skin irritation beneath the arrays.
When TTFields are delivered, the arrays may cause mild warming and tingling of the skin underneath them.
Please consult with a participating study physician at one of the centers for additional information about potential side effects from using NovoTTF-200(O), and the other treatments in the study [ENGOT-ov50/INNOVATE-3 Centers].
Do I need to carry the device all the time?
Patients may not necessarily be physically carrying the device most of the time, but will rather use it while it is continuously placed in one location (e.g. on a desk, a table or on the floor inside the carrying bag). The device was designed to allow the performance of normal daily routines. The device and the portable battery weigh about 1.3 kilograms, or 2.7 pounds altogether and can be carried in a dedicated shoulder bag or backpack when walking around.
Will the use of TTFields pose any risk to family members or other people?
There is no exposure of TTFields to non-users, such as co-workers and family members. The use of the device should not interfere with household or standard personal electrical devices.
How will the device affect my social life?
You can use the device while maintaining most of your daily routines. The arrays will be concealed underneath your clothes. The Device Support Specialist (DSS) will offer help and support related to appearance issues if you are using TTFields. For additional information, please see Living with TTFields.